The Challenge
Driven by technical, economic and institutional issues, critical drugs continue to experience supply shortages across the globe. While a number of regulatory and industry groups have been working diligently on mitigating root causes for these shortages, these issues continue to challenge the industry and the healthcare supply chain.
Drug manufacturers have recently begun to systematically apply best practices outlined by industry associations to their manufacturing networks and supply chains in an effort to assess and reduce risks to the supply, thereby assuring the uninterrupted availability of critical drugs to their customers.
In an effort to get ahead of this problem and avoid any potential for a drug shortage, a global supplier of critical medicines engaged xCell Strategic Consulting to complete a detailed assessment of the design, detail and thoroughness of its strategic manufacturing program on Drug Shortage Prevention and Business Continuity. The company's decision to select xCell was based on our expertise and previous experience in advising the International Society of Pharmaceutical Engineering (ISPE) on these issues.
The Solution
xCell Strategic Consulting believes the prevention of drug shortages has two major considerations:
-
Foundational elements need to be in place for a company to assure compliance with regulations and reliability of product supply.
-
Strategic decisions need to be made that provide guidance for a company to react and recover from any unforeseen event that could curtail production efforts and prevent product flowing from the plant.
Based on these overriding considerations, xCell developed a list of success factors to capture the critical elements that should be considered as a basis for the prevention of drug shortages. Our approach was used to assess the practices and the Drug Shortage Prevention Program developed at our client’s strategic manufacturing site.
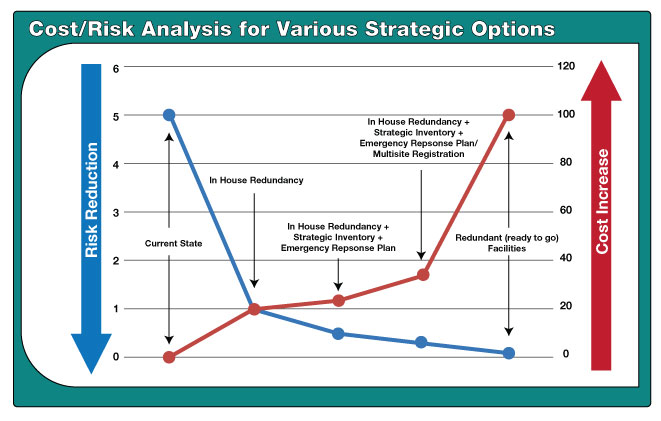
In achieving uninterrupted manufacturing supply, there is a broad spectrum of solutions a business must consider. Each solution will provide a level of risk mitigation but must also consider its ease of implementation, its ability to be available when needed and the cost to implement. Each set of solutions is based on a specific product, its unique manufacturing technology and supply chain considerations. By using a combination of supply chain management practices, redundant critical technologies and backup supply services; the industry has been able to achieve significant risk reduction as it relates to the potential for drug shortages. The figure below illustrates this cost/risk analysis for various strategic options.
The Result
Taking into account the list of factors and the guidance of the International Society of Pharmaceutical Engineers Drug Shortage Prevention Plan, the xCell team conducted a thorough review of the company’s strategic manufacturing facility, procedures and processes. In addition, the approach and thoroughness of the company’s quality systems and the foundational elements observed at the site were considered to be strengths in minimizing the risk of interruption in product supply.
The final xCell report outlines a comprehensive set of short, medium and long term recommendations that were adopted by our client. These strategies and practices, when fully implemented, will provide a robust system to ensure no shortage of any critical drug can occur within our client’s supply chain network.
For more detailed information on the specific non-confidential recommendations from this engagement, please contact xCell Strategic Consulting at our website links.



